Leading Providers of USP<797> Consulting, USP<800> Solutions, USP<797> Resources, USP Compliance, USP<800> Services




















Modular Cleanrooms knows cleanrooms can be constructed out of many varying materials from sheet rock to flexible vinyl, but no matter what material it is, the HEPA filter is the heart of every Cleanroom. That is why Modular Cleanrooms is a Master Distributor of Envirco Corp. and has the largest inventory in the central US of Mac 10 Fan Powered HEPA Filters. Along with the Mac 10 filters we stock several styles and sizes of cleanroom light fixtures (T8 with electronic ballasts) in standard Troffer and Flo-Thru styles, Suspended “T” Bar ceiling grid and ceiling tiles. We also stock replacement motors, pre-filters and HEPA filters (standard and RSR) for the Mac 10 series of Fan Filter Units (FFUs). Modular Cleanrooms is also able to purchase replacement HEPA filters directly from the manufacturers for most popular brands of FFUs.
The Econo-Tent softwall cleanroom is the most economical approach and design for your cleanroom needs. This design is commonly used in the microelectronics and medical mold injection industry as well as many others. Econo-Tent's softwall design allows it to be used as either a totally freestanding room or it can be used in combination with your existing walls and ceiling. Each room comes completely factory prefabricated for minimal on-site erection time and is totally self-contained with all components factory-finished.
Modular Hardwall Cleanrooms are prefabricated, factory engineered and designed to meet a full range of cleanroom requirements found in industries ranging from Micro-Electronics, Aerospace, Medical equipment and packaging. Modular Hardwall Cleanrooms are also an excellent product for the Compounding Pharmacy market. They meet and exceed all of the requirements found in USP 797.
The Aluminum Bio-Clean design emphasizes particle infiltration as well microbial contamination protection. The Aluminum Bio-Clean can be designed and certified to as clean as Class 10 (ISO 4) up to Class 100,000 (ISO 8) as well as Class 10,000 for USP 797. Each Aluminum Bio-Clean cleanroom can also be outfitted with Class 100 (ISO 5) work zones eliminating the need for (ISO 5) Clean Benches/Hoods.
The Micro-Clean cleanroom is specifically designed to meet requirements of the medical and pharmaceutical industries. Micro-Clean's design allows it to be used as either a totally freestanding room or it can be used in combination with your existing walls and ceiling. The Micro-Clean comes completely factory prefabricated for minimal on-site erection time and is totally self-contained with all components factory finished.
Visit our website for more information







Cleanroom Connection, your trusted pharmacy cleanroom supplier for over 20 years, offers a wide range of products for sterile compounding , pharmaceutical manufacturers, and healthcare facilities. Our products meet USP 797, USP 795, USP 800, and CGMP standards, ensuring compliance and safety. Our expert staff is here to assist you in selecting the right supplies and understanding protocols.
With veteran compounding pharmacist consultants boasting 35+ years of experience, we provide tailored solutions for pharmaceutical compounding, manufacturing, and hospital pharmacies. Benefit from exclusive pricing agreements with top manufacturers, guaranteeing wholesale prices and special offers. Take advantage of our lowest price guarantee and volume pricing contracts for added savings.
To setup an account or to get pricing, simply call us toll free at 800.616.5319
• Visit the Cleanroom Connection Website
• Play the Cleanroom Connection Informational Video
• View the Cleanroom Connection Platinum Pages Publication Ad



















Simplifi 797 is turnkey pharmacy compliance software that automates operations required to comply with USP chapters 795, 797 and 800. The customizable interface eliminates the paper and binder clutter of the inspection process, reducing time and effort for your team. We design the expert content, task management, and alerts to ensure you’re operating safely and compliantly—and we make them available on a mobile-friendly electronic platform, so you can provide the inspector everything they need at the touch of a finger.
Unlike other products that stop at inspection readiness, Simplifi 797 helps you continuously improve your practice with customizable analytics, up to 45 hours of continuing education and regularly updated best practices from industry experts.
Backed by Wolters Kluwer’s 200 years of leading domain expertise and a team of internal compounding compliance experts, Simplifi 797 handles the ‘what and how’ of USP Chapters 795, 797 and 800, so you can optimize operations, maximize staff competency, and pass every inspection with confidence.
The solution is custom-developed so each component aligns with the next, ensuring that everything from policies and procedures to training and task management are perfectly fit to your needs.
Streamline cleanroom operations and enhance compliance tracking documentation with task management, environmental sampling, master formulation templates and compounding logs. Easily manage multiple facilities from one centralized location.
The continued USP <795> and <797> revision conversations are drawing closer to finalizing the chapters, and the enforcement timeline is looming. The experts at Simplifi 797 are preparing so customers will be compliant at enforcement—we are enhancing our functionality and content to support the changes and developing resource documents to help you navigate the differences.
State-of-the-art analytics provide compliance activities and trends specific to your compounding sites, Quality Scorecard reports and more, to ensure a trouble-free inspection.
Staff receive ACPE-approved web-based training through an exlusive parternship with CriticalPoint that is updated as regulations evolve. Access expert-written observational competencies, an easy-to-use scheduler and a customizable competency builder.
When you purchase Simplifi 797, you're not just acquiring a quality management system. You're gaining a full support and success team dedicated to helping you get the most out of your compliance solution. First, our implementation team works with you to assess your situation, ensuring the solution is tailored specifically to your needs, and on your timeline. Once set up, you'll have a 24/7 support team for any ongoing assistance. Plus, your dedicated Customer Success Manager is committed to facilitating your compliance success.
Finally, the application and its expert content are updated quarterly based on customer feedback and industry trends, so you have access to the latest best practices. Wolters Kluwer's Simplifi 797 makes it easy to be compliant.
• Visit the Wolters Kluwer Health Website




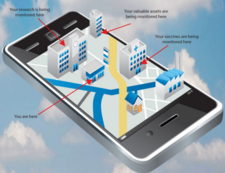
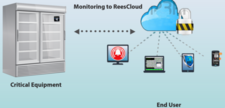
Rees Scientific specializes in protecting your valuable assets. Since 1982, Rees Scientific has been the industry standard for automated temperature monitoring. We offer continuous monitoring, centralized data collection, and simplified reporting. Monitor critical parameters such as temperature, humidity, differential Pressure, light control, etc. in vital equipment such as stability chambers, LN2 tanks, refrigerators, freezers, ultra-low freezers, incubators, etc. A single system can monitor a diverse facility with multiple locations while providing instant data access and sophisticated analysis right from your desktop computer, tablet or smartphone. Alarm notifications can be sent through telephone, text messages as well as e-mail.
View sensor conditions of your unit right from the Rees Scientific LCD display module. Ideal for pharmacies that house vaccines in their refrigerators. Monitoring temperature of vaccines is a crucial task that is regulated by many different government health organizations. The Rees Scientific LCD module complies with the guidelines of vaccines storage and handling from CDC, NIH, NIST, AAP, and PHAC.
Our LPA Cloud WiFi kits are a simple and economical way to monitor temperature in critical equipment such as refrigerators and freezers. The system will alarm out and notify when the temperature of a refrigerator or freezer fluctuates out of a given range. It is an ideal solution for laboratories, pharmacies, health clinics(Vaccine Temperature Monitoring) and food stores. Along with temperature, LPA Wireless also provides humidity and differential pressure sensors that help you become compliant with USP 797 regulatory requirements.
ReesCloud is an optimal asset that provides a secure, fast and easy way to continuously monitor your critical equipment and environment conditions. Features include:
Today, we continue to lead the field, taking cues from our clients and their environmental challenges. We ensure that your critical equipment and devices are monitored and maintained to set a standard every day without exception and without fail. Rees Scientific will continue to build products and services that help provide resolutions for our customers. Our goal is to be a quality provider of systems, services and solutions for our clients.
• Visit the Rees Scientific Website
• View the Rees Scientific Platinum Pages Publication Ad







Finding products that comply with USP <797> mandates isn’t always easy, but it is essential. And Health Care Logistics has you covered. We offer the most diverse selection of USP <797> and infection prevention solutions in the healthcare industry and we provide all the solutions you need in one convenient location.
Cleaning supplies such as Lint-Free Wipes and Sterile Cleanroom Wipes are great for cleaning lab equipment and medical instruments in areas where bacteria, particulate and extractable levels are critical concerns. Our Cleaning Tools & Supplies can help you achieve quick, effective cleaning and disinfecting in critical areas such as cleanrooms, isolators, restricted access barrier systems, filling lines, laminar flow hoods, cabinets, incubators and glove boxes. And our Infection Control Mats effectively remove dirt, dust and lint on contact from shoes, making it easier than ever to comply with USP <797> mandates!
Could your facility benefit from stainless steel storage containers, lock boxes and utility trays to simplify USP <797> compliance? Do you have an existing product you'd like to upgrade with a specially sized metal adaptor such as a bracket, plate, clamp or hanger that is USP <797> friendly? Whatever solution you seek, HCL can deliver. We create metal products to match your unique specifications so you can work more efficiently and produce better results. Our designers use stainless steel and aluminum, dependent upon your needs, and our design consultation services are always FREE!
• Visit the Health Care Logistics Website
• Play the Health Care Logistics Informational Video
• View the Health Care Logistics Platinum Pages Publication Ad



Founded in 1967 and exclusively devoted to the needs of the compounding pharmacist, at IMI creating products for the compounding pharmacist is our sole focus. As a result, our customers experience the quality, service, and value that only a specialized partner can offer. Through our partnerships with compounding professionals, we continue to advance our devices to serve the healthcare community. IMI’s customer-focused ethos and superior engineering capabilities allow us to be responsive to the needs of our partners and customers. Our capabilities and highly trained teams are the reasons we have remained the industry standard for tamper evident cap technology and how we continue to deliver customer-focused products to enhance pharmacy productivity, safety and security. All IMI products are manufactured in the United States at our FDA-registered, ISO 13485–certified facility under the strictest quality standards.
Compounded preparations are at their greatest risk when they leave the custody of your pharmacy. The benefits of tamper-evident products to address this risk have been recognized within the standards and guidelines of multiple influential organizations including the American Society of Health-System Pharmacists and the FDA. USP <797> Guidelines advocate “the use of tamper-evident closures and seals on CSP ports can add an additional measure of security to ensure product integrity regardless of the transport method used.”
Tamper Evident products increase overall accountability in the chain of custody of mediation, maintain sterility, prevent leakage, ensure patients receive the full intended dose and reduce the risk of contamination. Experts agree that the use of tamper evident products increases the confidence of pharmacies, health care workers, and patients.
IMI’s Prep-Lock™ Line of products provide high-value, high-quality, tamper-evident closures devices for a variety of drug delivery containers, including IV, Enteral, & Oral Syringes, Medication Cassettes, and IV Bags. Hospitals that partner with an outsource compounder, may already be familiar with Prep-Lock Tamper Evident Caps for IV Syringes. Over 85% of the top 503B compounding facilities trust IMI Tamper Evident Caps to secure their preparations. Incorporating these caps for in-house preparations can provide standardization of all syringes throughout the hospital. As a result, clinicians will not need training for administering syringes with multiple tamper evident methods. Tamper Evident Caps help to ensure the integrity of your compounds by providing a visual indication of tampering, misuse, or access. Installation and administration are highly efficient. Each sterile tray of 10 includes a unique keyed feature that allows the pharmacist to install a cap with a simple twist of the syringe; helping to reduce the risk of touch contamination and enhance aseptic technique. To administer, simply pull off the outer sleeve of the tamper evident cap and unscrew the remaining luer lock cap. Clinicians and pharmacists appreciate not having to deal with sticky tapes or frustrating shrink wrap. Tamper-Evident Caps represent a convenient and comparatively low-cost risk mitigation mechanism for pharmacies and health systems.
IMI’s Prep-Seal brand of products includes high-quality, American made caps and plugs for lure lock, luer slip and NRFit® devices. From their distinctive tray of 10 design that enhances aseptic technique to the unique color options, IMI works closely with their partners and industry professionals to continuously advance their devices to meet the needs of compounding pharmacists around the world.
IMI’s brand of Prep-Fill Adapters & Connectors provide pharmacists with feature enhancements to the sterile connectors essential to their everyday compounding processes. Their Guarded Luer Connectors offer a variety of connecting configurations, and features designed to enhance aseptic technique.
The RX-Vent family of products offers unsurpassed safety and efficiency to the pharmacist. The Chemo-Vent is used for reconstituting and withdrawing cytotoxic anti-neoplastic agents. It prevents blowback of chemotherapeutic and cytotoxic drugs during fill procedures. The Filtered Venting Needle is used for the sterile venting of drug vials. The provide pharmacist with economical solutions to enhancing safety in drug compounding procedures.
IMI’s RX-Tract brand of Needles provide health care professionals with unique length and gauge needles for compounding procedures. Available in lengths ranging from 3 inches to 8 inches RX-Tract Needles come in both blunt and sharp cannula types and are ideal for drawing liquid for reconstruction or to pre-fill syringes prior to administration.
FREE Sterile Samples are available for our entire product line.
See why IMI has become the brand pharmacists’ trust for innovative sterile products.
• Visit the International Medical Industries Website
• Play the International Medical Industries Informational Video
• View the International Medical Industries Platinum Pages Publication Ad
• View the International Medical Industries 20Ways Publication Profile
• Print the International Medical Industries Booth Information




Assessments
Regulatory compliance assessment includes regulatory requirements, professional standards of practice, accreditation standards, policies & procedures, and indicators representing high performing pharmacy operations.
Action Plans
Corrective action plan identifies resources, templates and best practices to improve probability of successful implementation and ongoing compliance.
Expert Support
Guidance by pharmacy practice regulatory specialist to advice, clarify, address barriers, promote and support continued improvement and compliance.
Set up your pharmacy for success.
From pharmacy set up to success, we are the partner that can help you with USP 797 and 800. We have expertise in all aspects from where your sterile compounding room should be to how it should be set up and managed. Should you undergo an audit, we can be your pharmacy point person and guide you through it.
CSP is the USP 797 & 800 expert and the trusted partner you need to set your pharmacy up for success.
• Visit the CPS Solutions Website
• Play the CPS Solutions Informational Video
• View the CPS Solutions Platinum Pages Publication Ad







Associates of Cape Cod, Inc. (ACC), is one of the world's largest manufacturers of products developed to detect and quantify gram-negative bacterial endotoxins and (1-3)-ß-D-glucans. Our products are used worldwide by leading pharmaceutical and medical device companies to ensure the safety of their parenteral drugs, biological products and medical devices. Our goal is to provide the best products and services, as well as the best technical support and customer service, in our industry to enhance the productivity and efficiency of all our customers. We are ISO 13485: 2003 certified, our laboratories are FDA Inspected and DEA Licensed and our Beacon Diagnostics ® laboratory is CLIA certified.
ACC is one of the world's largest manufacturers of products developed to detect and quantify gram-negative bacterial endotoxins and (1-3)-ß-D-glucans. Our products are used worldwide by leading pharmaceutical and medical device companies to ensure the safety of their parenteral drugs, biological products, and medical devices. Our Customer Service, Technical Service, and Contract Testing teams are ready to assist you with all aspects of endotoxin and glucan testing.
Pyroclear® Accessory Products
Pyroclear Pyroclear® brand accessory products are the first products in the industry that are certified to be free of interfering endotoxin and (1-3)-ß-D-glucan contamination. Pyroclear brand certified products include depyrogenated test tubes, 96-well microplates, pipette tips and LAL Reagent water. These products are designed to reduce Out of Specification (OOS) investigations due to contaminated accessories.
ACC is proud to offer a variety of instrumentation to support all your testing needs. Instruments like our Pyros Kinetix® incubating kinetic tube reader which offers the highest turbidimetric assay sensitivity in the industry. Bridge the gap between the affordability of filter-based readers and flexibility of monochromator-based systems with our VersaMax™ microplate reader.
ACC offers a complete suite of reliable, easy to use research products. Developed for research purposes only, ACC's Research Products are designed to improve pyrogen testing while offering our high standard of effectiveness.
Precise, easy-to-interpret data is an essential part of kinetic endotoxin detection programs. ACC offers three software packages designed to improve the efficiency and the compliance of your testing program all featuring 21 CFR Part 11 compliance, dynamic reporting options and increased data security.
• Visit the Associates of Cape Cod Inc Website
• View the Associates of Cape Cod Inc Literature
• View the Associates of Cape Cod Inc Platinum Pages Publication Ad
• View the Associates of Cape Cod Inc 20Ways Publication Profile







Element has three divisions: Laboratory Services, Validation & Calibration Services, and Manufacturing Services. We have expanded our services to offer biotechnology development services, such as master cell bank preparation. We offer services in molecular biology, virology, cell culture and analytical testing, training, validation and calibration services, HEPA filter certification, and scientific resources. We are GMP and FDA registered laboratory, and ISO 17025 accredited and 9001 compliant.
What makes us different?
Validation & Calibration
Our Validation & Calibration Division provides support services to pharmaceutical, biotechnology, medical device, and nutritional product companies. Our validation experts can provide comprehensive validation services that will meet your budget and timelines.
Manufacturing Services
We provide custom manufacturing services for the biotechnology, pharmaceutical, medical device, and other life science industries. We are FDA registered and ISO 17025 accredited. MQA is available 7 days a week to provide you with a fast turnaround time.
Additionally Offer
• View the Element Platinum Pages Publication Ad






For over 40 years, NuAire has consistently led the pharmacy compounding industry in safety standards and customer satisfac¬tion. We obsess over our customers’ needs and tailor unique solutions to meet those needs. With invaluable input from customers like you, we now offer a wide range of laminar airflow workstations (LAFWs), containment ventilated enclosures (CVEs), biological safety cabinets (BSCs), ultralow temperature (ULT) freez¬ers, Restricted Access Barrier Systems (RABS), and much more. With our cutting-edge robotic sheet metal facility, we can deliver you custom, innovative pharmacy compounding solutions in addition to the standard products that we sell.
The LabGard Class II Type A2 & Type B2 Biological Safety Cabinets (BSCs) effectively operate as Containment-Primary Engineering Controls (C-PECs) for sterile hazardous pharmacy drug compounding. C-PECs provide USP 800 compliant product, personnel, and environmental protection that can be externally exhausted after passing through HEPA filtration.
The PharmaGard Restricted Access Barrier System (RABS) series offers a physical barrier between personnel and product. The positive pressure Compounding Aseptic Isolator (CAI) protects the non-hazardous compounded sterile preparation inside, while our negative pressure Compounding Aseptic Containment Isolators (CACIs) offer containment for hazardous sterile preparations and a choice of a model that recirculates most air to the work zone or another that is total exhaust. NuAire offers accessories so that either of these CACI models can be connected to external exhaust in compliance with USP 800.
The AireGard Laminar Airflow Workstation (LAFW) series operates as a Primary Engineering Control (PEC) that complies with USP 797 by providing product protection by flushing the work surface with vertical or horizontal ISO class 5 air.
The LabGard NU-813 Containment Ventilated Enclosure (CVE) or Class I BSC also effectively operates as a C-PEC that complies with USP 800 for non-sterile hazardous drug compounding. The NU-813 offers personnel and environmental protection by creating a constant 80 fpm (0.41 m/s) air inflow that is exhausted through HEPA filters.
The Blizzard -86°C Ultralow Temperature (ULT) Freezer series provides consistent storage temperatures of -40°C to -86°C, ideal for vaccine storage, and are equipped with energy efficient compressor motors. All NuAire freezer models utilize environmentally friendly hydrocarbon (HC) refrigerants and vacuum insulated polyurethane (VIP) chamber walls. Available capacities range from 3.5 to 14.8 ft3 (100 to 828 L).
NuAire pharmacy compounding workstations can be fitted with an optional IV bar with 6+ SST hooks, mechanical auto-rising base stands, ergonomic arm attachments for computer monitor + keyboard/mouse, and glass sidewalls for added visibility.
• View the NuAire Platinum Pages Publication Ad








Personal protective equipment is the first line of defense against exposure to Hazardous Drugs and the most cost effective means to protect employees. Prudential Cleanroom Services' garments also protect the product from the employees. Humans are one of the largest contamination contributors to any cleanroom environment unless they are gowned correctly.
CLEANROOM GARMENT SYSTEM FEATURES
PCS VALIDATED REUSABLE MOP PROGRAM
Why purchase mop heads and dispose of them when you can reuse them?
All Prudential Cleanroom Services products are Validated SAL 10-6 for our Aseptic customers.
• Visit the Prudential Cleanroom Services Website
• View the Prudential Cleanroom Services Platinum Pages Publication Ad
• View the Prudential Cleanroom Services 20Ways Publication Profile






ChemoAlert is a surface sampling kit used by hospitals, pharmacies, infusion centers and anyone who handles chemotherapy drugs in the U.S. and Canada, to regularly test for drug residue. If gone unnoticed, workers may be exposed to antineoplastic drugs, which could be harmful to health over time. ChemoAlert was designed in compliance with USP <800> and NAPRA regulation, which drive the safe handling of hazardous drugs (HD) to help you safeguard your healthcare workers. To comply with these regulations, testing is recommended every 6 months in North America.
The easy-to-use kit, proprietary to Bureau Veritas, includes everything you need to collect a viable HD sample: swabs, vials, templates, wetting agent, gloves and an ice pack to maintain sample integrity during shipping and storage. After collecting samples from typical workplace surfaces, the swabs must be shipped to our AIHA LAP, LLC accredited laboratory for analysis, with results available within 10 business days. Rush results can be requested as quickly as the same day.
You can sample any workspace in a pharmacy, oncology clinic, infusion center, or hospital. Typical locations include benches, compounding areas, ante chambers, hoods, phones, balances, patient furniture, floors, bathroom facilities, cafeterias, and door handles.
A single swab can detect up to 19 HDs, including platinum-containing drugs:
As each kit includes 10 vials and swabs, you can detect a combination of up to 19 HDs across multiple surfaces using just one kit. With a detection limit of only 5 ng/sample, ChemoAlert also offers superior analytical sensitivity and specificity compared to similar products.
To utilize the ChemoAlert product, please follow these steps:
Step 1: Determine the number of locations and HDs for sampling. Click here for analysis pricing information.
Step 2: Purchase the ChemoAlert kit by clicking on the Order Now button below. The cost of the kit covers shipping and supplies needed.
Step 3: Once the kit is received, place the ice pack in the freezer and sample the locations using the provided wetting solvent and media.
Step 4: Pack up the samples, ice pack and Chain of Custody and send the cooler back to the lab using the prepaid shipping label.
Want to test for drugs that are not on the list? No problem. We have analytical methods available to test for 1000+ drugs individually, although some restrictions may apply. Please refer to our Pharmaceutical Fee Schedule for more information.




Baker has a long history of providing the highest quality containment and clean air products for retail, hospital and clinical pharmacy applications. Our products continue to set the industry standard for safety, performance, operating efficiencies and ergonomics through innovative research, meticulous engineering and on-going testing.
With pharmacy laws, guidelines and regulations targeting the preparation of sterile injectable drugs, clean air and containment equipment is playing an increasingly critical role in day-to-day pharmacy operations. Laminar-flow clean benches, gloveboxes and biological safety cabinets are required in-house elements for minimizing potential contamination and exposure during compounding.
For 60 years The Baker Company has been helping our customers advance science, discovery, and clinical care by providing the right equipment to meet their needs. We look forward to working with you!
We are committed to helping you protect your patients and employees, and offer a full range of products to help you be compliant with USP <795>, <797>, and <800> including:
PHARMACY GLOVE BOXES
Positive pressure sterility assurance barrier glove box for complete product protection when compounding non-hazardous pharmaceutical compounds and related clinical, pharmacy and process applications.
For sterile compounding of hazardous or potent pharmaceutical compounds, chemotherapy agents and IV admixtures that can be harmful to pharmacy personnel.
BIOSAFETY CABINETS
The most energy efficient, comfortable and safe A2 cabinet in the industry, the SterilGARD e3 is designed for sterile product preparation and biological experimentation involving agents of low to moderate risk. Also available is the compact model SG-303, a 3-foot biosafety cabinet with a smaller footprint for low-volume facilities.
The BioChemGARD® e3 is a breakthrough in total-exhaust biological safety cabinets. Engineered specifically for laboratories that need containment and removal of vapors, mists and particulates, the BioChemGARD e3 has a revolutionary airflow system that increases users’ comfort and productivity while decreasing the overall operating costs. Designed for biological testing and product preparation involving low to moderate risk agents where chemical effluent is present and clean air is essential.
LAMINAR FLOW CLEAN BENCHES
With a spacious work area and brightly illuminated for maximum productivity and unique high velocity air return slots, EdgeGARD® HF Clean Bench offers both user comfort and product protection. Horizontal and vertical flow models are available.
OPTIONAL REMOTE COMPOUNDING VALIDATION PACKAGE
Phocus Rx remote compounding validation system from Grifols is a “plug & play” solution that provides a safe, reliable and easy-to-use environment to document and validate the compounding of prescription IV drugs. The system improves quality assurance and workflow efficiency by helping to ensure that medications are prepared correctly and labels are accurate. Baker biosafety cabinets and clean benches are available fully integrated with Phocus Rx.
• Visit the The Baker Company Website







As a premier developer of innovative medical devices, our greatest priority is making the lives of healthcare professionals easier while improving patient outcomes. Amongst the many divisions we excel in, Parasol specializes in a portfolio of products which includes competency testing, bonded antimicrobials, daily disinfectants, and lint-free wipes for the cleanroom pharmacy space.
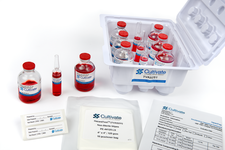
Quality assurance products – packaged in a lint-free thermo-form container and built to comply with USP <797> and <800> requirements. Cultivate products are designed for air particle monitoring, surface testing, personal aseptic technique testing, needleless dispensing, safe handling of hazardous drugs and more.
A patented, EPA-registered antimicrobial treatment clinically validated to help reduce the spread of infection and cross-communication of dangerous microorganisms on surfaces. MicrobeCare’s™ fast mode of action effectively destroys organisms from the treated surface without dissipation.
Pharmacy Options:
• View the Parasol Medical Platinum Pages Publication Ad




Esco Lifesciences is a world-leading life science company with a diversified portfolio and sales in over 100 countries. As a manufacturer of laboratory and biopharma equipment, and IVF medical devices, Esco offers tailored solutions that fit the needs of laboratories in various industries.
Esco Lifesciences contributes to meet the challenges of the 21st century with a diverse range of business units. The company continuously innovates its products to help the clinical and industrial laboratories achieve successful conclusions in research and development, quality control, and analysis. We continue to provide reliable world-class equipment to help pharmaceutical companies make their products safer and more cost-effective
Time is running out, but you can rely on Esco for a one-stop shop for equipment to comply to USP 800.
USP 800 is officially enforced by local, state, and federal regulatory agencies that require compounding pharmacies to comply, or otherwise face a hefty fine or even closure. Hazardous drugs must be compounded in a proper containment primary engineering control (C-PEC) such as biosafety cabinet (BSC) or compounding aseptic containment isolator (CACI) that is externally vented and located in an ISO Class 7 cleanroom.







When it comes to your refrigerator and USP <800>, Helmer is here to help. Cold storage is an important part of keeping your hazardous drugs safe and stable. Improper cold storage can lead to unnecessary waste, financial loss, lack of compliance, and pose major safety concerns. As we get closer to July of 2018, facilities will continue to adapt their hazardous drug room to meet USP <800> compliance. Helmer upright, undercounter, and countertop refrigerators are safe and effective cold storage solutions for your sensitive and expensive hazardous drugs.
GX Solutions Professional Medical-grade Refrigerators
Proper cold storage of medications and vaccines is critical in the healthcare environment, and often can be the difference between viable, effective products and ineffective or wasted product. With GX Solutions, professional, medical-grade cold storage is now available and takes cold storage to new levels. Focusing on temperature, noise and energy management, GX Solutions are the first cold storage solutions to optimize all three areas and offer a professional, medical-grade solution.
GX Solutions pharmacy and vaccine refrigerators are powered by OptiCool™ technology which pairs variable capacity compressor (VCC) technology and natural hydrocarbons (HC) to achieve performance characteristics that offer a superior storage environment. Optimized temperature management (uniformity, recovery and stability) provides confidence that contents are stored at the right temperature regardless of their location within the unit with fewer temperature deviations. In addition, noise levels are lowered, energy usage is reduced, and environmental sustainability initiatives are supported.
GX Undercounter Pharmacy Refrigerators
GX Upright Pharmacy Refrigerators
Pass-thru Refrigerators
Pass-thru refrigerators create a boundary between clean rooms and regular facility environments while permitting products to be removed from outside the clean room on a “first-in-first-out” basis, improving inventory control and boosting productivity.
Laboratory Freezers
Laboratory freezers are designed specifically for the high demands of healthcare. Built-in monitoring systems provide continuous monitoring, real-time alarm notification, and status notification for worry-free storage, allowing your staff to focus on the critical functions of their job.
Undercounter
Upright
• Visit the Helmer Scientific Website
• View the Helmer Scientific Platinum Pages Publication Ad





Since 1959, Mystaire has been the manufacturer at the forefront of environmental safety and air pollution control products. Ductless fume hoods are a safe and cost-effective alternative for protecting laboratory workers from hazardous materials. In order to ensure maximum protection, Mystaire manufactures each ductless fume hood to the highest standards in the industry.
The Latitude Series C Filtered Hoods provide biotechnology and pharmaceutical companies with an important safety solution when handling potentially harmful chemicals in particulate and/or liquid forms. Latitude maximizes user accessibility and incorporates redundant filtration for added safety and easy maintenance. This hood was designed with rear wall pre-filter and HEPA filtration. This design pulls potentially harmful particulate away from the operator’s breathing zone in an even horizontal airflow path, increasing particulate and vapor capture. Standard on each Latitude is two power cord access ports, a waste attachment port and the dark blue base for work surface contrast. Our Saf-T-Zone™ filtration system is incorporated into the Latitude, isolating the pre-filter and main HEPA filter during filter change. This allows our fume hoods to continue to operate and provide containment during main pre-filter and HEPA filter replacement.
The Mystaire® CleanPrep Plus+ Horizontal laminar flow clean bench is designed to provide an ISO 5 particle-free clean-air environment for laboratory testing, sample prep, and analysis, manufacturing, inspection, or pharmaceutical procedures. Precise horizontal laminar airflow is obtained through a high-quality HEPA filter installed in the rear baffle of the cabinet. The microprocessor controller constantly monitors the airflow and condition of the HEPA filter. The microprocessor controller monitors filter life and alerts the end user if airflow is sacrificed, and filter change out is necessary. Air is forced through the HEPA filter and then enters the chamber through a diffuser that distributes clean ISO 5 particle-free clean-air over the entire work surface.
The work surface on each Mystaire CleanPrep Plus+ Horizontal Laminar Flow Clean Bench is constructed from structural white polypropylene for easy cleaning and decontamination. The clean bench can be placed on an existing countertop, or used with an optional powder-coated stand with level feet or a polypropylene base cabinet. The solid, polypropylene work surface combined with the ultra-smooth horizontal laminar airflow creates an ideal environment when handling critical component inspections, assembling optical components, or working with pharmaceutical ingredients.
The Mystaire® CleanPrep™ BSC is designed as a Class II Type A2 biological safety cabinet for applications that require both operator protection from potentially harmful biological samples and process protection to minimize potential sample contamination from the environment. This is achieved by recirculating 70% of the cabinet air through ULPA filters while exhausting 30% of the air through ULPA filtration.
CleanPrep BSC is designed to provide product, personnel, and environmental protection through the combination of unidirectional downflow ULPA-filtered air with high-velocity inflow room air to create an active air barrier. The active air barrier at the front of the cabinet separates the laboratory from the work area and maintains a constant inflow speed to prevent environmental contaminants from entering the work area, or potentially harmful contaminants from escaping.
CleanPrep BSC cabinets come standard with a stainless steel bar IV bar and ten hooks to allow for clean and efficient IV bag preparation. CleanPrep BSC cabinets are ideal for applications that require USP 797 compliance.
Mystaire offers complete containment solutions for general chemistry, molecular biology, pharmaceutical, forensic, and academic research, and manufacturing. Let us help you maintain USP 800 compliance and clean working areas.




For over 20 years, Q.I. Medical has been a trusted provider of quality assurance products to compounding pharmacies throughout the world. Our focus is on developing
easy-to-use products for our customers to help them with their in-house testing needs. Whether it is a low, medium, or high risk facility, Q.I. Medical can help them to be in compliance with regulatory guidelines (USP <797>, USP <71>, 503a or 503b, etc.).
We offer unique products for pharmacists and nurses who handle sterile solutions. Our focus is on devices, test kits, and accessories that improve aseptic technique.
Applications include:
• Visit the QI Medical Website
• View the QI Medical Platinum Pages Publication Ad



With a vast range of reusable garments and products, ISO Certified processing / available sterilization, and personalized service from your dedicated sales service representative, partner with Cintas Cleanroom to keep your controlled environment Ready for the Workday®.
Whatever your contamination control requirements, Cintas has a range of garments and products in a variety of fabrics to help you manage your controlled environment.
Get the right Coverall for your specific controlled environment, delivered by Cintas.
Frock options to keep your employees in the cleanroom Ready™.
A variety of options to keep you covered head-to-toe.
Anti-static garments for semiconductor and electronics applications.
Head-to-toe FR gowning solutions to help protect both the wearer and environment.
The right footwear for your controlled environment.
Mops and cleaning products to help you keep your cleanroom clean.
Thousands of disposable cleanroom products, delivered to you and conveniently ordered and managed by your service sales representative on each service delivery.
• View the CINTAS Platinum Pages Publication Ad





California-based corporation was formed to supply quality products for the biotechnology and healthcare industries in the United States.
As a manufacturer, a manufacture representative, and a wholesaler, we offer an extensive array of products, including:
ISO-MED provides an unparalleled level of convenience for our customers. As a result, we offer a unique opportunity to provide top-quality products, responsive customer service, and highly cost-effective purchasing options for all our customers.
• View the ISO-MED Platinum Pages Publication Ad





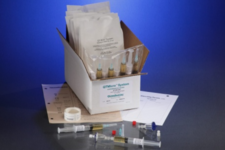
Acute Care Pharmaceuticals is located in San Diego, California. We are a full-line pharmaceutical wholesaler and USP <797> & USP <800> compliant cleanroom disposables manufacture. Since our founding in 1997, we have been continually licensed by, and in good standing with, the California State Board of Pharmacy.
For over 20 years Acute Care Pharmaceuticals has been servicing the healthcare industry by delivering exceptional products and services to healthcare providers nationwide. We offer a wide range of USP <797> and <800> compliant disposable products that are manufactured under the highest standards. Our USP <797> compliant product line includes sterile alcohol, non-linting wipers, disinfectants, gloves, media test kits, apparel, and more.
Looking toward the future and developing products that exceed your expectations is our number one goal. Our new USP <800> line of products was developed with you in
mind, because your safety and the safety of your employees matter.
USP 800 Products include:
ISO Cleanroom Standards
Acute Care Pharmaceuticals meets ISO Class 5 standards on all of our wipers and ISO Class 5-6 on our new Pharma-Sat™ Canisters. This demonstrates our commitment to quality in the design and manufacturing of its cleanroom disposables.
With an excess of 20 GPO and IDN contracts, our goal is to make your buying experience simple and easy. Our Pharma-Choice™ Brand of products are stocked at AmerisourceBergen, Cardinal, and McKesson, where you can receive your special discounted contract pricing.
If you want quality products and customer service that you can count on, call us today at 1-888-909-7700 for more information and your GPO contracted pricing.
• Visit the Acute Care Pharmaceuticals Website
• View the Acute Care Pharmaceuticals Platinum Pages Publication Ad
• View the Acute Care Pharmaceuticals 20Ways Publication Profile






Eagle, established in 2003, began as a dedicated partner addressing regulatory needs in the compounding industry. Over the years, it has evolved into a premier scientific solutions provider, with a distinguished FDA-registered CGMP testing/calibration laboratory spanning over 80,000 square feet. Equipped with state-of-the-art instrumentation, cutting-edge technologies, and advanced methodologies, Eagle delivers tailored, high-quality scientific solutions designed to meet the most rigorous industry standards.
Eagle is uniquely positioned to serve a diverse range of clients, including:
We proudly serve clients throughout North America and globally.
Eagle offers a comprehensive range of scientific solutions. Our laboratory provides analytical chemistry and microbiological testing services for raw materials, medical devices, and finished and compounded pharmaceutical products.
Eagle is the only lab in the USA offering three alternative rapid sterility tests in addition to traditional USP 71 sterility testing, making us an industry leader in rapid sterility testing. With its patent for testing oil-based compounded products on ScanRDI®, Eagle provides a fully compliant 1-day turnaround rapid sterility test. We help our customers reduce quarantine time by up to 90% with these advanced alternative methods.
Our team of experts also handle projects for:
Our consulting team provides customized GAP audits, due diligence audits, regulatory responses, SOP development, environmental monitoring solutions, designated person competency assessment and any other needs upon request. In addition, our engineering specialists excel in facility design, cleanroom certifications, smoke studies, equipment calibration and equipment qualification (IQ/OQ/PQ).
As part of our comprehensive Environmental Monitoring (EM) solutions, we provide TSA (Tryptic Soy Agar) plates in two convenient sizes, designed for both personnel monitoring and surface sampling. In addition to our high-quality plates, we offer a range of complementary services to support your monitoring needs:
To verify the efficacy of cleaning programs, we offer SurfaceShield, a wipe sampling kit designed to detect both hazardous and non-hazardous drugs on surfaces. This versatile and cost-effective kit supports achieving and maintaining USP <800> compliance and meeting Title 21 Part 211 CFR requirements. Additionally, we test for the majority of drugs listed in the NIOSH guidelines.
These integrated services ensure precise and reliable results, helping you maintain the highest standards of quality and compliance.
With our broad expertise, Eagle is your all-in-one solution provider.
• Visit the Eagle Analytical Services Website
• View the Eagle Analytical Services Platinum Pages Publication Ad








At AirClean® Systems, our goal is to continually offer the safest and most reliable ductless fume hoods, workstations, and enclosures in the industry. Since 1992, we provide eco-friendly lab equipment that protects the operator or process. Every product is made and thoroughly tested in-house at our manufacturing facility located in Creedmoor, NC.
Incorporated in each product is our state-of-the-art automatic safety controllers and monitoring systems. These constantly monitor the airflow and systems and are designed to alert the user immediately of any adverse conditions. The controllers were designed to be user-friendly for ease of understanding while using them with the product.
AirClean Systems PowderSafe™ enclosures are the solution for the weighing of potent compounds and chemicals. PowderSafe ventilated enclosures offer both particulate and liquid chemical filtration capabilities. Engineered for safety and balance stability, each PowderSafe balance enclosure is constructed from materials that offer exceptional durability as well as ease of cleaning.
Our Type A enclosure is the compact, mobile solution engineered specifically for balances. This provides the ideal weighing environment for toxic compounds and/or liquid chemicals. Included is the AirSafe® Automatic Safety Controller technology for airflow and filtration monitoring.
Our type B enclosure effectively weighs powders to seven decimal places. The polypropylene construction provides the mass and vibration resistance crucial for accurate weighing. Equipped with our user-friendly AirSafe® Automatic Safety Controller, this provides airflow and filtration monitoring systems.
Our Type C enclosure incorporates the HEPASafe™ Technology and airflow dynamics from our Type B along with fume containment capabilities of our ductless fume hoods. This product is perfect for weighing powders and solvents due to the thermally-fused polypropylene construction. AirSafe® Automatic Safety Controller is our user-friendly monitoring system included in the equipment for airflow and filtration.
AirClean Systems focuses on helping you fulfill the USP obligations with the proper enclosures to use that include the redundant HEPA filtration. All solutions are available in a wide array of sizes with customization capabilities to fit specific lab work space.
• Visit the AirClean Systems Website
• View the AirClean Systems Platinum Pages Publication Ad






Particle Measuring Systems (PMS) partners with pharmaceutical aseptic and controlled manufacturing customers to deliver the knowledge, technology, quality, and service needed to meet global regulatory requirements and ensure the highest product quality. We offer complete contamination monitoring solutions, beginning with risk assessments from our Advisory Services team, followed by data collection using our world-class viable and non-viable particle counters, and continuing through compliant data management, analysis, and reporting. With deep expertise in contamination control, we help you achieve compliance in manufacturing and successful product release. As one of the world’s leading experts in cleanroom contamination control, we provide fully integrated, robust monitoring solutions, including:
We also offer a wide variety of expert Advisory Services including:
Additionally, PMS offers a suite of purpose-built environmental monitoring solutions to help pharmacy compounders comply with regulations and streamline their contamination control workflows. Innovative tools like the Lasair® Pro portable particle counter, MiniCapt® Mobile portable microbial monitor, and fully compliant DataAnalyst™ software, can reduce both resources and downtime.
Founded over 50 years ago, PMS is a trusted thought-leader in Pharmaceutical clean manufacturing and other Life Science industries. Globally regarded as a thought leader in contamination monitoring and regulatory compliance, our team provides multiple webinars, papers, and training throughout the year to support cleanroom professionals and manufacturers with reliable guidance on the application of, and compliance to, global regulations.






Charles River
Chapter <797> of the United States Pharmacopeia (USP) outlines the facility and operational requirements for compounding sterile drugs and specifically calls for the collection and monitoring of environmental data through air and surface sampling...
CPS Solutions
Regulatory bodies are paying increasing attention to hospital and health system pharmacies regarding sterile medication compounding...
CPS Solutions
Comprehensive Pharmacy Services drives compliance with an end-to-end process: complete assessment, strategy development and implementation...















































Connect with thousands of pharmacy professionals throughout every practice setting.
The server is experiencing a problem at this time. Try again later.